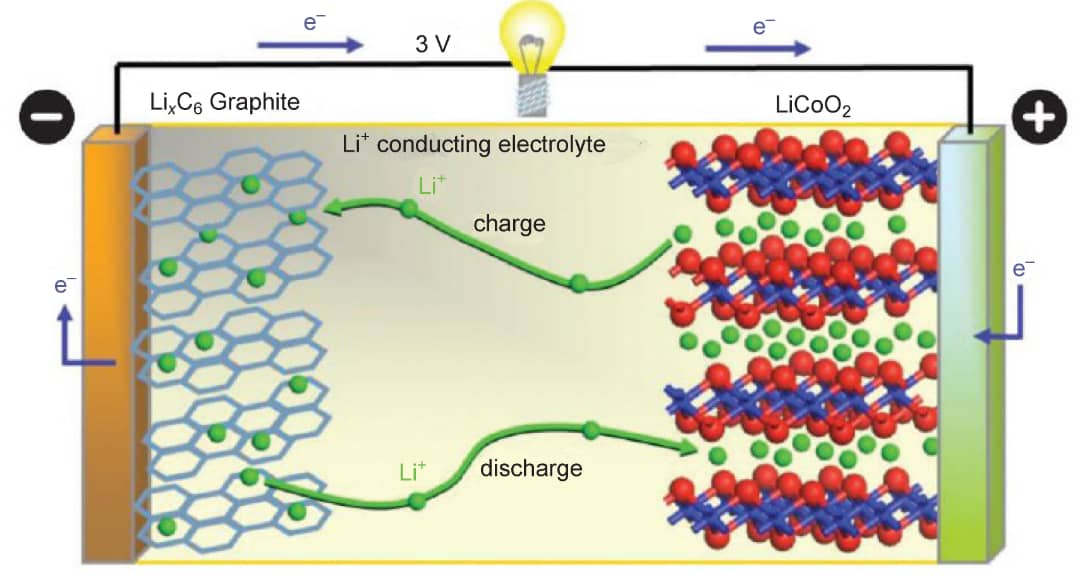
Lithium-ion Battery
A lithium-ion battery is the foremost rechargeable battery for today’s applications. It has three important parts which are cathode, anode and the electrolyte. The cathode is the most important part of the Li-ion battery. This is because the energy of the battery comes from the cathode. The cathode is mainly made up of lithium transition metal oxides. The cathode must be an intercalation material. Only certain structures of materials exhibit the intercalation behaviour. They are namely, the layered compounds, the spinels and the olivines.
Nanomaterial Research group focuses more on the cathode from layered and spinel compounds. Some of the cathode materials that were synthesized are compounds with novel chemistries such as LiNixCoyO2, LiNixCoyMnzO2, LiMn2O4, LiMnxTiyO4, etc. There are several synthesis methods that are used at the Centre to synthesize the cathode materials. They are the self-propagating combustion, the sol-gel and solid-state reaction methods. The characterizations of the cathode materials are extensively done using Simultaneous Thermogravimetric Analysis (STA), X-Ray Diffraction (XRD), Field Emission Scanning Electron Microscopy (FESEM), High Resolution Transmission Electron Microscopy (HRTEM) and X-Ray Photoelectron Spectroscopy (XPS). The cells are tested in terms of their charge-discharge profiles, cycling behaviour, rate-capability, etc.