Aiming Towards Co-free Ni-rich Cathodes for Next-gen Rechargeable
Lithium-ion Batteries
|
For the Fourth Industrial Revolution (IR 4.0), our living style and working atmosphere are bridging between the big data and advanced technologies that leading for the advancement of artificial intelligence, data analytic, matrix city, i-device, i-transportation, smart robotic and others. Energy transformation will play a key role in IR 4.0. World nowadays face challenges in terms of resources, environment and energy. Leading the way in energy transformation that will impact the whole society is automobile. Currently, there is a rapid progress on electric vehicles production by automotive industry such as Tesla, BMW, Volkswagen and NiO. The production of electric vehicles indicating that the world has started undergoing energy transformation from conventional combustion engine that depend on fuel to move to the free fuel vehicles that can move using a battery. Battery technology that has been used in current electric vehicles is rechargeable Li-ion batteries (LIBs). This lightweight, rechargeable and powerful battery is now used in everything from mobile phones to laptops and electric vehicles. It can also store significant amounts of energy from solar and wind power, making possible a fossil fuel-free society. By 2025, one of Nobel Laurette in chemistry 2019, Professor Akira Yoshino predicted that the future car is artificial intelligence electric vehicle (AIEVs). According to him, to reach that point, EVs need to reach new mileage of beyond 500 km/charge and has durability up to 5000 cycles. Currently, EVs only have a driving range of ~400 km/charge with 1500 cycles. To achieve such vision, Prof. Yoshino said that battery energy densities, durability and cost need to be improve further. It is necessary to design a promising rechargeable battery that can be sustained for a long period of time and recharge/discharge efficiently. Ultimately, LIBs have no doubt as energy storage device and power source due to high energy densities and long-life cycle. However, future improvement in terms of energy density, cycle life, and safety can be made to meet the growing demands of energy storage. |
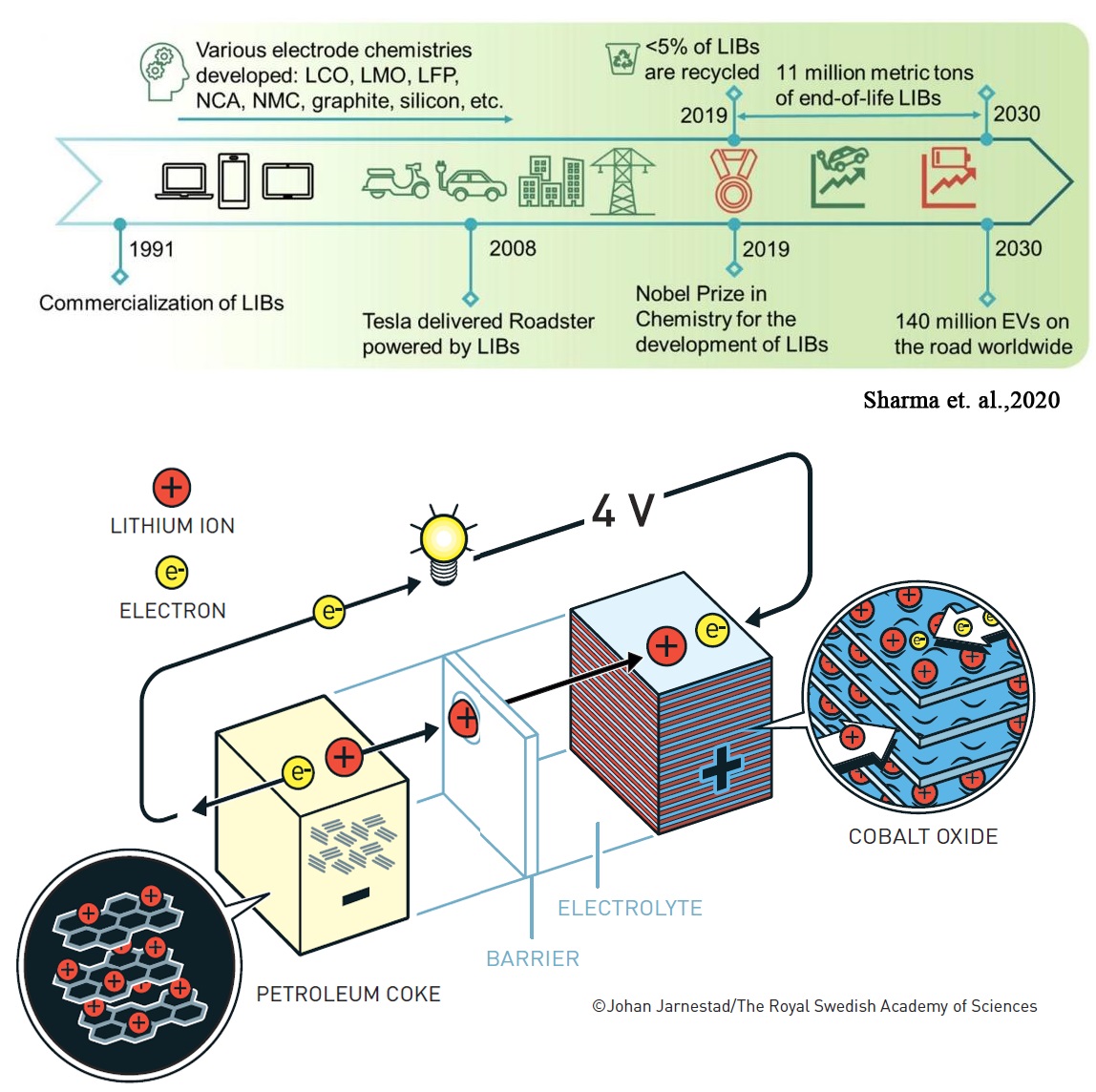 |
| In LIBs, the cathode materials hold the specific capacity and long-life cycle of the battery. The pursue of high energy density of LIBs, however, it comes with an elevated instability of cathode materials, causing a major concern on the durability and safety of the LIBs. Back then 1990s, LiCoO2 is the first successful commercialized cathode materials in LIBs which held practical capacity of ~140 mAhg-1 and has been used widely in our electronics devices until present. However, for EVs, extremely high power required has limits LCO application due to LCO has lower thermal stability and Co been an expensive metal resulting intolerable for EVs production. Thus, a new chemistry of cathode materials that has similar or exceed electrochemical performance with lower production cost than LCO has been develop. Intensive studies have been reported toward the formulating of multi component elements in the conventional LiCoO2 cathode materials, LiCo1-xMxO2 (M= Ni and Mn) which give a new layered cathode material, Li[NixCo1-2xMnx]O2 and Li[Ni1-x-yCoxMny]O2 (NCM). Note that transition metals are beneficial for these new layered structures in which the Ni2+ ions act as an active species, Mn4+ ions stabilize the crystal structure and the Co3+ ions reduce the cation mixing between Ni2+ and Li+ ions. Combination of Ni, Co and Mn ions in the layered R-3m structure can retain the advantages of high capacity LiNiO2 and excellent rate capability of LiCoO2 as well as less expensive and less toxic than the commercialized LiCoO2 system. At an operating voltage of 4.3 V, specific capacity of NCM with different combination of Ni, Co and Mn can increase up to 160 mAhg-1 for NCM111, 170 mAhg-1 for NCM 532, 180 mAhg-1 for NCM622 and 200 mAhg-1 for NCM811 compared to LCO which only held ~140 mAhg-1. It is important to notice that increase Ni in NCM cathode materials resulting in increasing specific capacity. However, high Ni content incurs severe capacity fading during cycles. Till now, cathodes with Ni content less than 60 % are fully commercialized and this is still not good enough for the EVs industry where they need high energy density of NCM811. Now, our group in Centre for Functional Materials and & Nanotechnology (CFMN), are tried to design a new Ni-rich cathode material that has less or zero cobalt content with has excellent electrochemical performances, but greener and cheaper production cost compared to the existing cathode materials. To know more about this work, please do feel free to contact us, muhdfir@uitm.edu.my. | |
|
|
|
